Study of reactions modified by vibrational strong coupling published in PCCP
- Sketch of SN2@Si reaction and reactant vibrational modes.
Our work “On the SN2 reactions modified in vibrational strong coupling experiments: reaction mechanisms and vibrational mode assignments” has just been published in Physical Chemistry Chemical Physics.
In recent years, several experimental works have reported modified kinetics of thermal chemical reactions when vibrational modes of reactants were under vibrational strong coupling (VSC) to Fabry-Pérot cavity modes. These results are very encouraging for future applications of polaritonic chemistry, as they could provide new means to catalyze reactions without any external driving, i.e., by simply placing the reactants between two mirrors (or letting them flow between them). However, despite several efforts in the community (including us), there is still no theory capable of explaining these experiments, with two particularly puzzling aspects: First, that there is an effect under collective strong coupling (where many molecules are coupled to each cavity mode and the per-molecule coupling is small) and second, that it only works if the cavity modes and molecular vibrations are in resonance (i.e., their frequencies match).
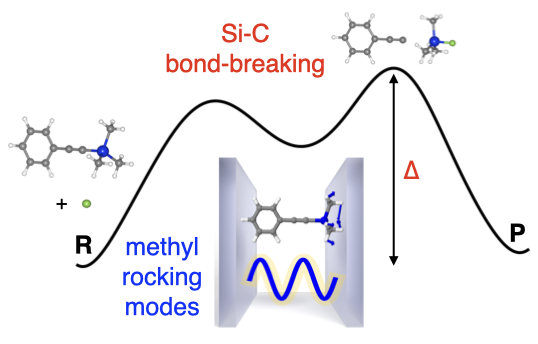
The first reactions whose rate was modified under VSC were SN2 nucleophilic substitution reactions on silicon centers, in two papers by the Ebbesen group in Strasbourg (in Angewandte Chemie and in Science). These reactions are not as common as the well-studied ones involving a nucleophilic attack on a carbon center. Previous work on similar reactions has shown that the reaction profile is highly dependent on the central atom and nearby substituents. This motivated us to study the reaction mechanism of these SN2@Si reactions in normal conditions, i.e., outside a cavity, in order to provide a firm base for further studies of their modification under VSC.
We first performed electronic structure calculations for these two reactions and found that the rate-limiting step of these SN2@Si reactions is due to cleavage of either the Si-C or Si-O bonds. Interestingly, in the experiments, the reaction rates were only modified when the cavity modes where on resonance with molecular absorption lines that were identified with the stretching vibrations of these two bonds. We performed a detailed analysis of the vibrational modes of the reactants, which showed that the mode assignments in the original experimental works had to be modified. In particular, the strongly coupled modes actually correspond to vibrations that are highly mixed among different fragments. Since we do not have a theory explaining how reaction rates can be modified under VSC yet, our results are of fundamental importance for the current phenomenological understanding of these experiments. They imply that the mechanism might not be as simple as a “direct” VSC-induced modification of the bonds that break.
For more details, please see the full paper (Open Access). Furthermore, Clàudia recently gave a webinar explaining these results together with our previous works related to this subject published in Physical Review X and in Angewandte Chemie.
 MMUSCLES
MMUSCLES